Bafna Pharma’s shares are listed in Bombay Stock Exchange ltd., Mumbai. (BSE Code: 532989)
Bafna Pharma’s corporate office is located at Chennai. The two factories are also located in Chennai
one at Madhavaram and other at Grantylon. The R&D facility is also located at Grantylon.
The markets that the company serves are categorized as Exports. The export market is in turn
categorized as ‘Regulated market’ and ‘Non-regulated Market or Emerging Markets’. Bafna Pharma is
present in the regulated market of the United Kingdom and Emerging Markets of Asia, Africa, and
Australia.
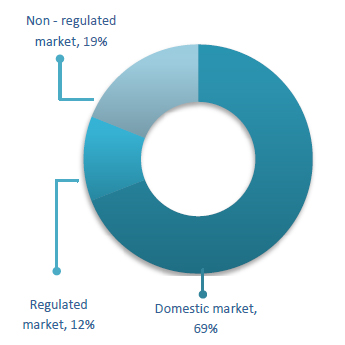
The total revenue achieved by the company is Rs. 7600 lakhs in FY ‘10. The company’s revenue from UK
market is Rs. 891.3 lakhs during the year, a 700% growth compared to the revenue from the same market
in FY 09. This signifies the shift in company’s focus towards high margin markets. The revenue share
from this market has grown from about 2% of the total sales during FY ‘09 to 12% during FY ‘10.
The Company plans to maintain and grow on this high margin growth trajectory.
At present, the R&D unit established is focussed into development of formulations. The key services
are
- Product development of Generics drugs specially for regulated markets like Europe, US and Australia
- Technical analysis of formulations
- Product development for market
- Dossier filing for both regulated and Non regulated markets
The company plans to hive off this unit to extend the services to other companies and to make the division a profit center.
Company has two manufacturing facilities in Chennai one at Madhavaram and Grantlyon.
- The Madhavaram facility is earmarked to cater to the requirements of non - regulated markets while the Grantlyon facility caters to the exports to the regulated market.
- The Grantylon facility specializes in manufacturing of Non-Betalactam products in solid oral dosage.
- The facility is accredited with EU - GMP, UK - MHRA, TGA Australia approval.
No facility is USFDA approved as of now. But the company has plans to obtain US FDA approval also.
The company has not paid any dividend as it plans to conserve the funds to support the working capital
and capital expenditure requirements of the fast propelled growth.